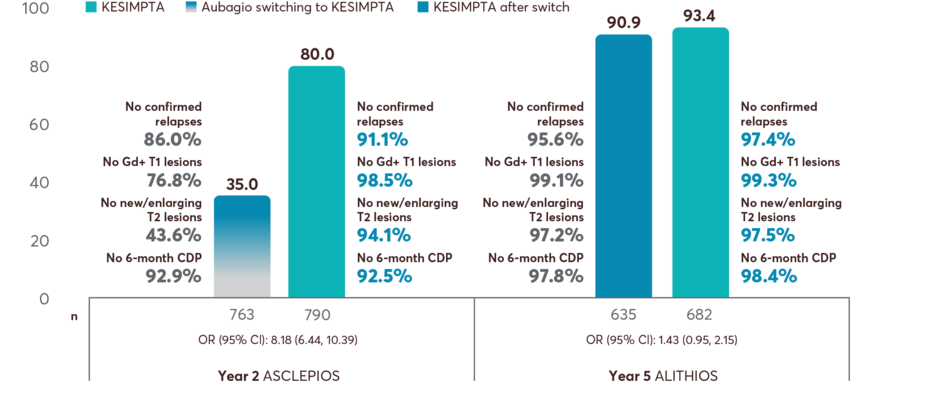
POST HOC ANALYSIS
In ASCLEPIOS I and II vs Aubagio® (teriflunomide): Primary end point, ARR 51% (0.11 vs 0.22), 58% (0.10 vs 0.25). Key secondary end points: number of Gd+ T1 lesions per scan 98% (0.01 vs 0.46), 94% (0.03 vs 0.52); annualized rate of new or enlarging T2 lesions 82% (0.72 vs 4.00), 85% (0.64 vs 4.16); 3-month CDP 34% (10.9 vs 15.0).1