Targeted and precisely delivered B-cell therapy
Watch the mechanism of action video
This animated video brings the immune system to life with a molecule's eye view of KESIMPTA
KESIMPTA® (ofatumumab) mechanism of action
KESIMPTA is thought to work by selectively binding to sites on both the small and large extracellular loops of CD20.1
KESIMPTA causes CD20+ B cells to be vulnerable to both immediate and delayed B-cell lysis by mechanisms such as complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity1
B cells in the lymph nodes
Preclinical studies showed that SC delivery preferentially targeted B cells in the lymph nodes2-4
B cells in the spleen
SC delivery of KESIMPTA is thought to spare B cells in the spleen to help maintain immune function, based on preclinical evidence5
The precise mechanism by which KESIMPTA exerts its therapeutic effects is unknown.
The clinical relevance of these data is unknown.

DIGITAL EDUCATION LAB
Watch a video with an expert perspective on the KESIMPTA mechanism of action
Targeted and Precisely Delivered Therapy:
Understanding KESIMPTA Mechanism of Action
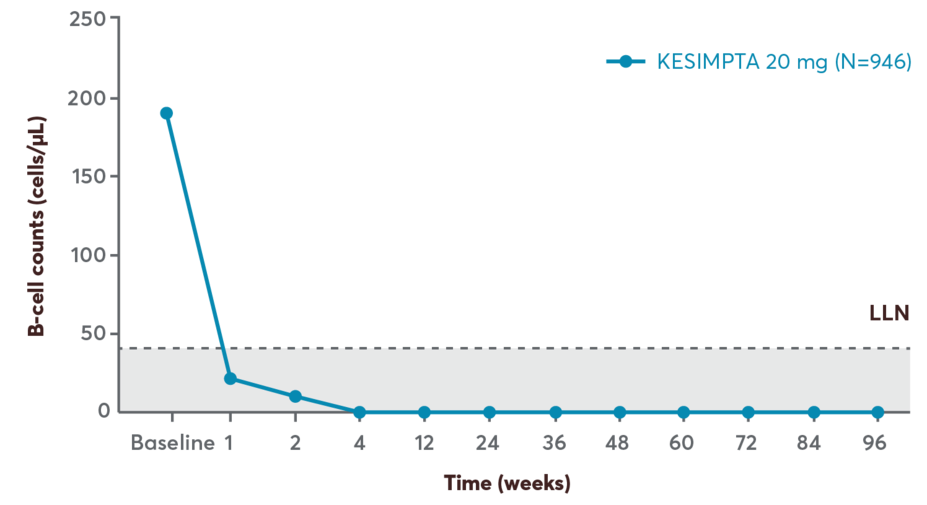
Rapid and sustained B-cell depletion
Subcutaneous, targeted, and precisely delivered therapy that provides rapid B-cell depletion6,7
B-cell depletion sustained over the dosing period
Reduction in B cells was seen as early as 1 week after treatment initiation2
B-cell counts remained below LLN for 97% of patients in ASCLEPIOS I and 92% of patients in ASCLEPIOS II from 12 weeks through 120 weeks2
B-cell repletion as early as 6 months after treatment discontinuation2
Data from ASCLEPIOS I and II indicate a median time of 24.6 weeks to B-cell recovery (either to LLN or baseline value) post treatment discontinuation
Modeling and simulation for B-cell repletion corroborate these data, predicting median time to B-cell recovery of 23 weeks post treatment discontinuation

"When my neurologist provided the data for me for KESIMPTA, it gave me hope."
Kristin: mom, real estate agent, KESIMPTA patient
Actual patient taking KESIMPTA who was compensated for time. Individual results may vary.
LLN, lower limit of normal; MOA, mechanism of action; SC, subcutaneous.