Vaccination of infants born to mothers treated with KESIMPTA during pregnancy1*†
In infants of mothers treated with KESIMPTA during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts. Depletion of B cells in these infants may increase the risks from live or live-attenuated vaccines‡
Inactivated vaccines may be administered, as indicated, prior to recovery from B-cell depletion, but an assessment of vaccine immune responses, including consultation with a qualified specialist, should be considered to determine whether a protective immune response was mounted‡
Currently available COVID-19 vaccines on the market, by Pfizer/BioNTech, Moderna, and Janssen, are non-live vaccines.3
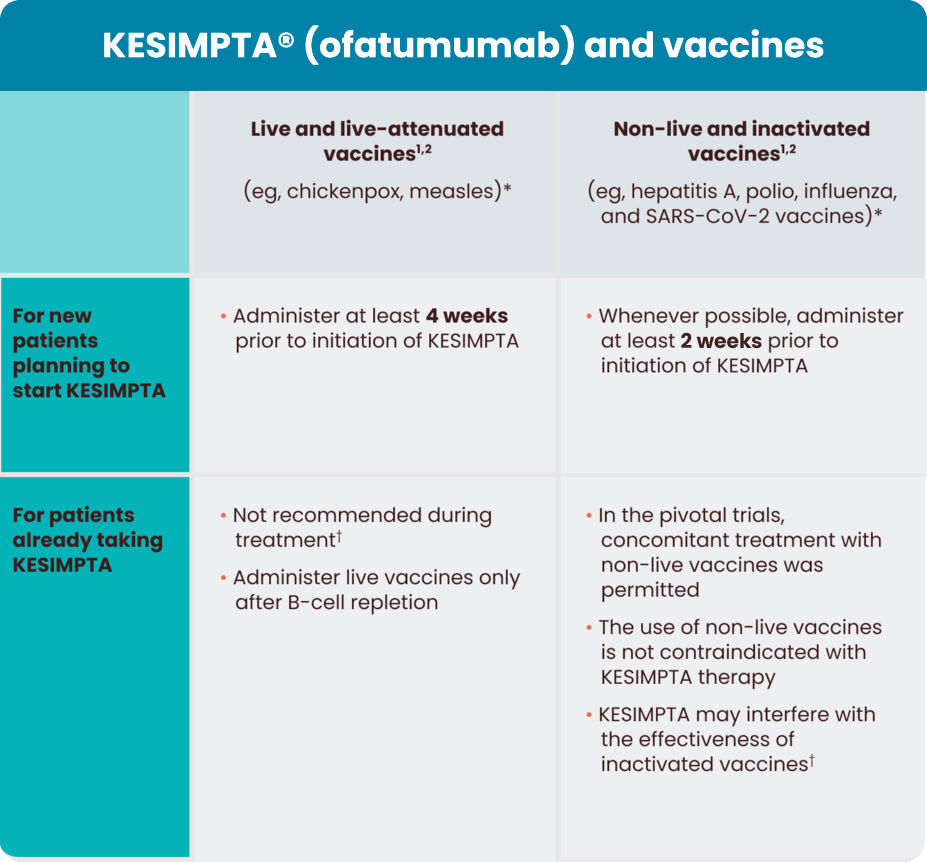
Any vaccine used in patients taking KESIMPTA should be administered in accordance with the KESIMPTA full Prescribing Information.
Immune response
B cells are part of a complex immune response to both viruses and vaccinations4,5
T cells are also involved and play a fundamental role in viral infections by helping B cells produce antibodies. They also orchestrate the response for other immune cells. Some T cells kill infected cells to reduce the viral burden4,5
Data from a relapsing MS clinical study also showed that T cells remained largely unaffected in KESIMPTA-treated patients. T cells are an important part of a response to viral infections, including COVID-195,6
KESIMPTA is a targeted and precisely delivered B-cell therapy
When delivered subcutaneously, KESIMPTA is thought to promote preferential depletion of B cells in the lymph nodes7
Preclinical evidence suggests that KESIMPTA may spare B cells in the spleen that help maintain immune function8
The precise mechanism by which KESIMPTA exerts its therapeutic effects is unknown.
Read more on KESIMPTA vaccine-related information
*As of March 2021, KESIMPTA has not been studied with vaccines.
†Post vaccination, an assessment of vaccine immune responses, including consultation with qualified specialists, should be considered to determine whether a protective immune response was mounted. It is unknown whether KESIMPTA may interfere with vaccine efficacy.
‡Data from Studies 1 and 2 indicate a median time to B-cell recovery to either LLN or baseline value of 24.6 weeks post treatment discontinuation. PK and PD modeling and simulation for B-cell repletion corroborate these data, predicting median time to B-cell recovery to LLN of 23 weeks post treatment discontinuation.
LLN, lower limit of normal; MS, multiple sclerosis; PD, pharmacodynamic; PK, pharmacokinetic; SC, subcutaneous.