Demonstrated safety and tolerability profile of KESIMPTA® (ofatumumab)
.
Pivotal Trial Safety
KESIMPTA has an established safety profile comparable to Aubagio® (teriflunomide), an oral therapy
Adverse events
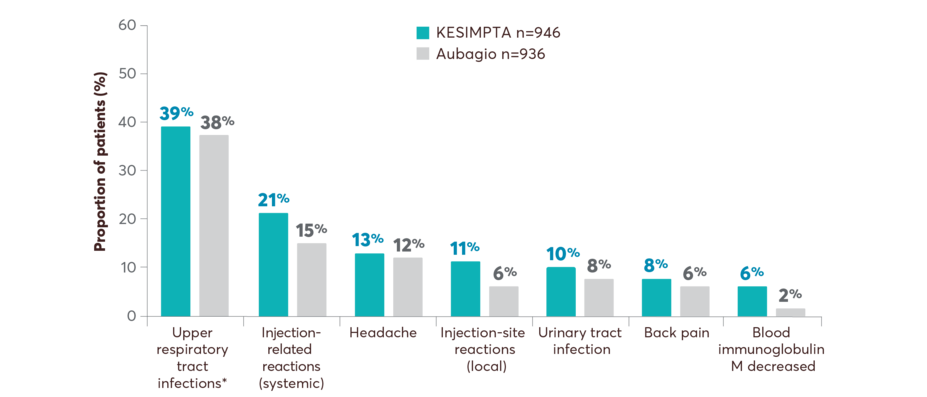
Safety across pooled ASCLEPIOS I and II studies1
*Includes the following: nasopharyngitis, upper respiratory tract infection, influenza, sinusitis, pharyngitis, rhinitis, viral upper respiratory infection, tonsillitis, acute sinusitis, pharyngotonsillitis, laryngitis, pharyngitis streptococcal, viral rhinitis, sinusitis bacterial, tonsillitis bacterial, viral pharyngitis, viral tonsillitis, chronic sinusitis, nasal herpes, tracheitis.
Rate of infections
The overall rate of infections and serious infections in patients treated with KESIMPTA was similar to Aubagio (51.6% vs 52.7%, and 2.5% vs 1.8%, respectively)1
Treatment discontinuations
Pooled data from both clinical trials show that treatment discontinuation rates due to adverse events were similar between KESIMPTA (5.7%) and Aubagio (5.2%)2
The most common cause of discontinuation in patients treated with KESIMPTA was low IgM (3.3%), defined in trial protocols as IgM at 10% below the LLN1
Treatment-induced ADAs were detected in 2 of 914 (0.2%) KESIMPTA-treated patients; no patients with treatment-enhancing or neutralizing ADAs were identified1
Patients who took Aubagio in the trials received placebo injections. Over the trial period, systemic and local injection reactions were reported in 21% and 11% of patients with KESIMPTA compared to 15% and 6% of patients treated with Aubagio who received matching placebo injections, respectively1
Dig deeper into long-term data
Tolerability
Demonstrated tolerability profile: systemic injection-related reactions (IRR)
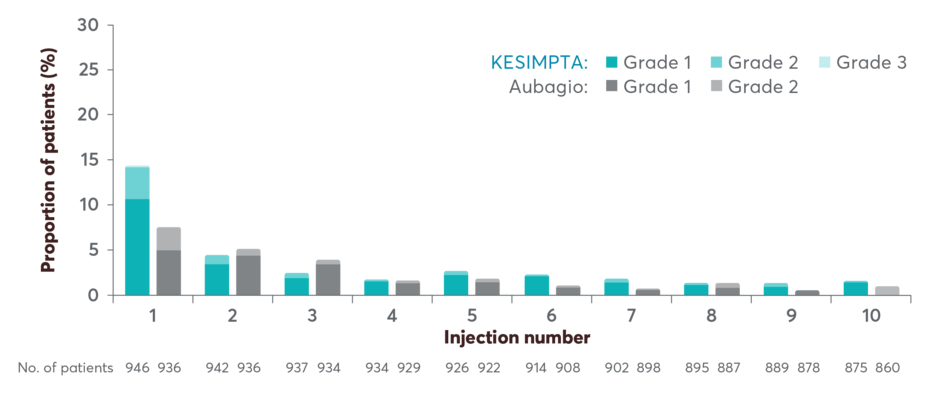
Systemic IRRs by injection in the ASCLEPIOS trials1,2,4
Injection-related reactions occurred in <3% of patients after the 3rd dose1
The incidence of systemic IRRs was highest with the first injection (14.4%), decreasing with subsequent injections (4.4% with second, <3% with third injection)
Two (0.2%) patients treated with KESIMPTA reported serious IRRs
The most frequently reported symptoms (≥2%) included fever, headache, myalgia, chills, and fatigue
Patients who took Aubagio in the trials received placebo injections. Over the trial period, systemic and local injection reactions were reported in 21% and 11% of patients treated with KESIMPTA compared to 15% and 6% of patients treated with Aubagio who received matching placebo injections, respectively

Long-term Safety
KESIMPTA has a well-established safety profile demonstrated over 7 years5
Safety profile through year 75
Click to Enlarge
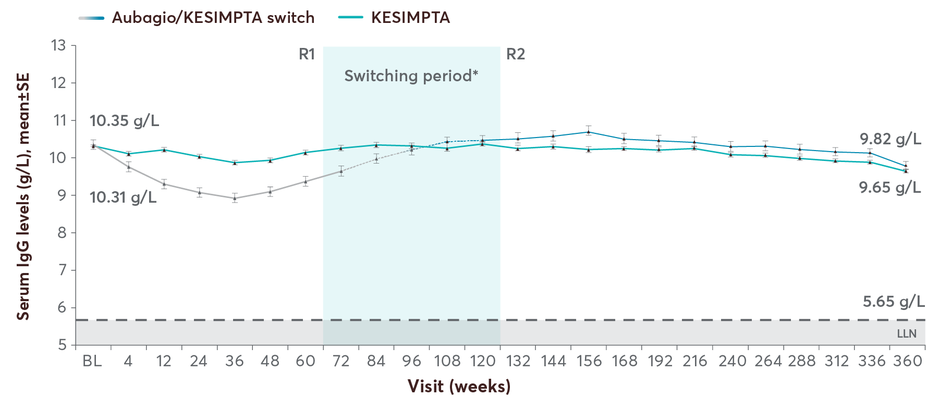
Mean IgG levels remained >LLN (5.65 g/L) in 96.8% of participants at all assessments5
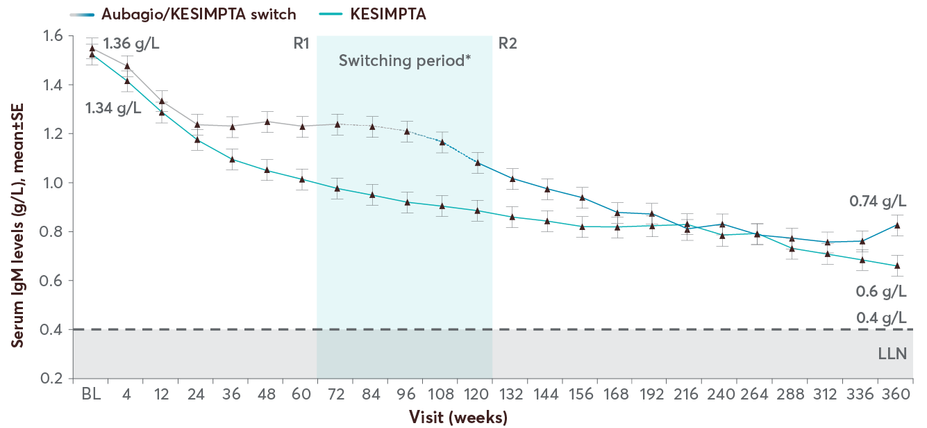
Mean IgM levels decreased but remained >LLN (0.4 g/L) and were >LLN at all assessments in 64.5% of participants5
The proportion of participants with Ig-related AEs leading to KESIMPTA interruption or discontinuation due to low IgG was 0.2%/0.2% and for low IgM was 10.4%/3.6%5
ALITHIOS study design: ALITHIOS is an open-label, umbrella extension, Phase 3b, single-arm study evaluating long-term (up to 7 years) data. The primary objective was to assess safety and tolerability (N=1969) in patients with RMS who had at least 1 dose of KESIMPTA (20mg SC) (including drop outs) from the ASCLEPIOS I/II, APLIOS, and APOLITOS trials. APLIOS (N=284) was a phase 2,12-week, open-label, parallel-group study. APOLITOS (N=62) is a phase 2, 24-week, double blind, placebo-controlled, parallel-group core-part followed by an open-label extension-part for up to 48 weeks, study in Japan and Russia. The secondary objective was to assess efficacy (N=1367) in patients with RMS who had at least 1 dose of KESIMPTA (20mg SC) from the ASCLEPIOS I and II trials who continued or switched to KESIMPTA treatment. A long-term (up to 7 years) analysis (n=465) was conducted to evaluate efficacy in the recently diagnosed treatment-naïve subgroup treated with KESIMPTA.5-10
No conclusions of clinical outcomes can be drawn.
*Included participants receiving ≥1 dose of KESIMPTA in ASCLEPIOS I/II, APOLITOS, APLIOS, or the alithios extension study.5
†Including the following: sudden death (n=1), esophageal adenocarcinoma (n=1), completed suicide (n=1), COVID-19/COVID-19 pneumonia (n=1), COVID-19 (n=2), gastric ulcer perforation (n=1), COVID-19 pneumonia (n=1), intestinal metastasis (n=1), COVID-19 pneumonia/pneumothorax (n=1), pneumonia/septic shock (n=1), and injury (n=1).5
AE, adverse event; Ig, immunoglobulin; IgG, immunoglobulin G; IgM, immunoglobulin M; LLN, lower limit of normal; SAE, serious adverse event.
![“My doctor and I chose KESIMPTA for my first RMS treatment. I felt comfortable knowing it has a safety profile demonstrated over 7 years.” – Caylee R. [25] years old. Volleyball Coach, Outdoor Enthusiast. Started KESIMPTA first line in 2022](https://usim.beprod.kesimptahcp.com/sites/kesimptahcp_com/files/styles/hero_full_width_width_2560/public/2025-06/2.0-caylee-quote-desktop.jpg?itok=m6xAyRUS)
Give your patients confidence with evidence on long-term safety.5 Start them on KESIMPTA today
Immune System Maintenance
A B-cell therapy that maintains long-term immune function
Patients on KESIMPTA maintained stable mean IgG levels for up to 7 years; mean IgM levels declined but remained above LLN5
.
Mean IgG levels with KESIMPTA up to 7 years5
The majority of the patients had Ig levels above the LLN: 96.8% for IgG and 64.5% for IgM5
No clinically meaningful association was observed between IgG/IgM levels <LLN and risk of serious infections5
Serious, including life-threatening or fatal, bacterial, fungal, and new or reactivated viral infections have been observed during and following completion of treatment with anti-CD20 B-cell depleting therapies.
Mean IgM levels with KESIMPTA up to 7 years5
In the Phase 3 ASCLEPIOS trials, 14.3% of patients treated with KESIMPTA experienced a decrease in IgM1
In the long-term analysis, mean IgM declined over time but remained above the LLN5
Read more on immunoglobulins
Components of immune function may be maintained in KESIMPTA patients
.
The precise mechanism by which KESIMPTA exerts its therapeutic effects is unknown. The clinical relevance of this data is unknown.
In the extension analysis (ALITHIOS), the overall incidence of serious infections was low5
Incidence of serious infections was consistent with that in the Phase 3 ASCLEPIOS I and II trials and did not increase with treatment up to 7 years despite the COVID-19 pandemic5
The most frequently reported serious infections were COVID-19 (2.59%), urinary tract infection (1.01%), lower respiratory tract infection (excluding COVID-19; 0.96%) and appendicitis (0.76%)18
KESIMPTA has the potential for an increased risk of infections, including serious bacterial, fungal, and new or reactivated viral infections; some of these infections have been fatal in patients treated with other CD-20 antibodies.
Ig testing and monitoring can help manage increased risk of serious infection
Monitor the level of immunoglobulins at the beginning, during, and after discontinuation of treatment with KESIMPTA until B-cell repletion
Consider discontinuing KESIMPTA if a patient develops a serious opportunistic infection or recurrent infections if immunoglobulin levels indicate immune compromise
No increased risk of severe or serious COVID-19 outcomes in patients taking KESIMPTA over 5 years in the ALITHIOS trial19†
COVID-19 related data—Analysis of data from December 2019 to September 25, 2022
- 648 (38%) of the 1703 patients (as of September 25, 2022) enrolled in ALITHIOS who received KESIMPTA reported that they had contracted COVID-19
- COVID-19 cases with severity, seriousness, outcomes, vaccination status, and breakthrough infections were all included in this analysis
- The open-label extension study is not blinded and not controlled
- 50 cases were considered serious with 82% recovery rate at the cutoff; there were 5 fatal outcomes
- Fatal outcomes (5/648 or 0.8%) due to COVID-19 in KESIMPTA-treated patients were lower than those reported in the general population (2.1%)20
- Three of these patients were unvaccinated; 2 patients were fully vaccinated‡
- The 5 fatal cases consisted of the following: COVID-19 (n=2), COVID-19 pneumonia (n=1), COVID-19 and COVID-19 pneumonia (n=1), COVID-19 pneumonia and pneumothorax (n=1)
No statistical or clinical conclusion can be made.
†Results from an analysis of data from the open-label, long-term extension Phase 3b study (ALITHIOS) from the start of the COVID-19 pandemic in December 2019 to September 25, 2022. As of September 25, 2022, 648 of the 1703 patients enrolled in ALITHIOS who received KESIMPTA reported that they had contracted COVID-19 (confirmed [n=603]; suspected [n=45]).19,20
‡Fully vaccinated means at least 14 days after completing the primary vaccine series, may or may not be after booster. These 2 fatal cases occurred before booster: one case had multiple risk factors for severe COVID-19 and the other case was complicated by a bilateral pneumothorax.19
Learn more about KESIMPTA and Vaccines